"Informed AI News" is an publications aggregation platform, ensuring you only gain the most valuable information, to eliminate information asymmetry and break through the limits of information cocoons. Find out more >>
Breakthroughs and Challenges of the New Antiretroviral Drug Lenacapavir for HIV
- summary
- score
Gilead Sciences recently announced that its HIV drug lenacapavir, in phase III trials, demonstrated 100% efficacy among 2,134 cisgender women with no cases of infection. Lenacapavir is an injectable capsid inhibitor designed to impede the assembly of the virus's protein shell and has been approved in the United States for HIV treatment, requiring combination with other antiviral drugs.
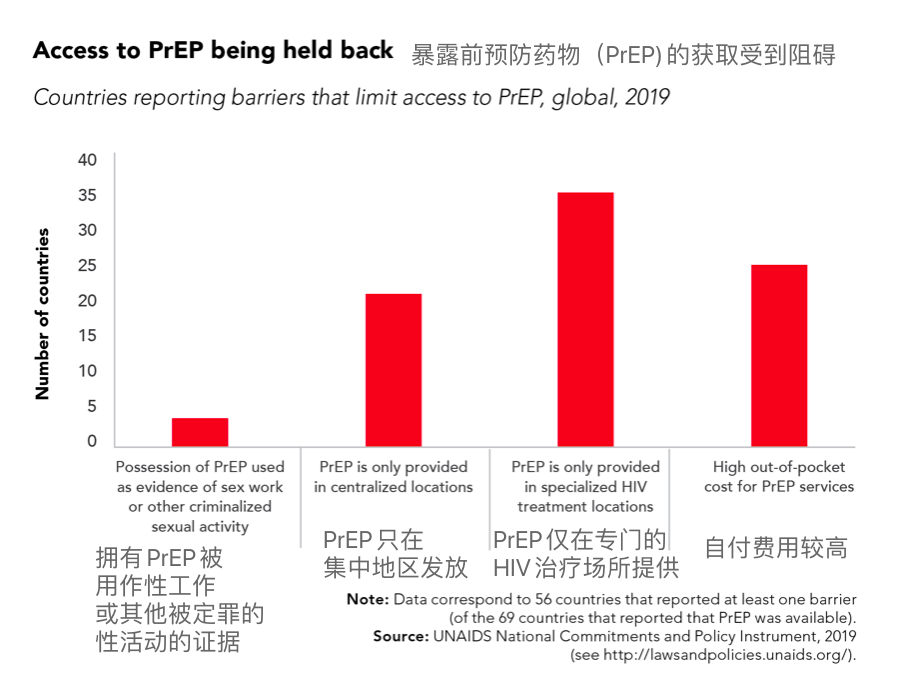
The drug has shown excellent performance in preventing HIV infection, with Gilead's CEO calling it a new tool for HIV prevention. However, lenacapavir faces multiple challenges in ending HIV transmission: a large global population of HIV-infected individuals, high costs of preventive medications, and the virus's strong mutation capabilities.
Despite these challenges, lenacapavir's long-acting properties (requiring only two injections per year) bring hope to HIV patients, potentially alleviating the psychological burden of daily medication, improving mental health, and reducing HIV transmission.
Gilead has a long history in antiviral research, having previously launched a hepatitis C drug that increased cure rates to nearly 100% and contributed to a shrinking market size. The success of lenacapavir not only boosted Gilead's stock price but also added another achievement to its efforts in HIV prevention and treatment.
Overall, while lenacapavir brings new hope to HIV prevention and treatment, completely ending the HIV epidemic will require further research and efforts.
| Scores | Value | Explanation |
|---|---|---|
| Objectivity | 6 | 内容客观,全面报道,深入分析。 |
| Social Impact | 5 | 引发广泛社会讨论,影响公众意见。 |
| Credibility | 5 | 完全可信,权威来源,证据充分。 |
| Potential | 5 | 几乎必然触发更大事件。 |
| Practicality | 4 | 高度实用,可直接应用于实际问题。 |
| Entertainment Value | 2 | 略显单调,包含少量娱乐元素。 |